Thermodynamic Machines: An Evolutionary System as a Designer of Future Environments
The proposed machine looks to use global warming´s temperature increase, greenhouse gases emissions, and technological mechanisms as inputs, in order to create a large-scale reversible thermodynamic machine that is able to accelerate or decelerate the evolutionary processes, generating unexpected hybrid environments.
The proposed thesis looks to make contributions in the domains of knowledge by analyzing global warming in the condition of Hyperobject. According to Timothy Morton, member of the object-oriented philosophy movement, hyperobjects are objects massively distributed in time and space relative to humans; they are all related to some other entity, whether or not they are directly manufactured by humans. He defines the main characteristics of hyperobjects, stating that these “are, which means that they ‘stick’ to beings that are involved with them. They are nonlocal; in other words, any ‘local manifestation’ of a hyperobject is not directly the hyperobject. They involve profoundly different temporalities than the human-scale ones we are used to. In particular, some very large hyperobjects, such as planets, have genuinely Gaussian temporality: they generate spacetime vortices, due to general relativity. Hyperobjects occupy a high-dimensional phase space that results in their being invisible to humans for stretches of time. And they exhibit their effects interobjectively; that is, they can be detected in a space that consists of interrelationships between aesthetic properties of objects1.”
Timothy Morton considers global warming as the prime example to identify hyperobjects. But what do we understand by Global Warming? According to the Oxford Dictionary, it can be interpreted as a progressive increase in the comprehensive temperature of the earth´s atmosphere due to the “greenhouse effect” caused by increased levels of some gases. These gases, including water vapor (H2O), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), ozone (O3), chlorofluorocarbon (CFC), and hydrofluorocarbon (HCFC, HFC), allow sunlight to pass through the atmosphere where it is absorbed by the earth´s surface. The earth´s surface then radiates it back to space in the form of heat; but part of the heat gets trapped in the atmosphere because of the presence of the gases2.
Global warming presents some characteristics that define Morton´s idea of hyperobject. It is viscous in the sense that it “burns the skin on the back of my neck, making me itch with physical discomfort and inner anxiety3.” This is evidence that global warming affects all beings involved with it. It is also nonlocal, meaning it is not visible to humans, if not is visible as a collusion between objects affected by it. “When you feel raindrops, you are experiencing climate, in some sense. In particular you are experiencing the climate change known as global warming. But you are never directly experiencing global warming as such4.” It is massively distributed in time and space (temporal undulation) in ways that bewilder humans beings, and makes interacting with them complicated and mysterious. It is phased; we can only see pieces of it at a time because of its trans-dimensional quality. “We only see pieces of them at once, like a tsunami or a case of radiation sickness. Less than adequate perception of higher dimensions of structure, which is where the hyperobjects live5.” In order to see global warming, humans would have to occupy a trans-dimensional space to see it “unfolding explicitely”. Finally, it is interobjective. This means it is formed by relations between several objects. Hyperobjects leave footprints; earthquakes can be considered footprints of global warming. They originate because of a variation in oceanic temperature, which change the pressure on Earth’s crust and provoke landslides, liquefaction, and/or tsunamis.
As mentioned before, Global Warming is the result of a process by which radiation from the Earth´s atmosphere heats the crust. It is produced by human activities, and this heat excess impacts the planet Earth in different ways:
• Sea Level Rise: increased by 4mm per year. The rate in the last two decades is nearly double that of the last century6.
• Actual Temperature: increased by 1ºC since the Industrial Revolution. Most of the warming occurred in the past 35 years, with 16 of the 17 warmest years on record occurring since 20017.
• Shrinking Ice Sheets: Greenland lost 150 to 250 cubic kilometers (36 to 60 cubic miles) of ice per year between 2002 and 2006, while Antarctica lost about 152 cubic kilometers (36 cubic miles) of ice between 2002 and 20058.
• Ocean Acidification: acidity of surface ocean waters has increased by about 30 percent since the Industrail Revolution (the amount of carbon dioxide absorbed by the upper layer of the oceans is increasing by about 2 billion tons per year)9.
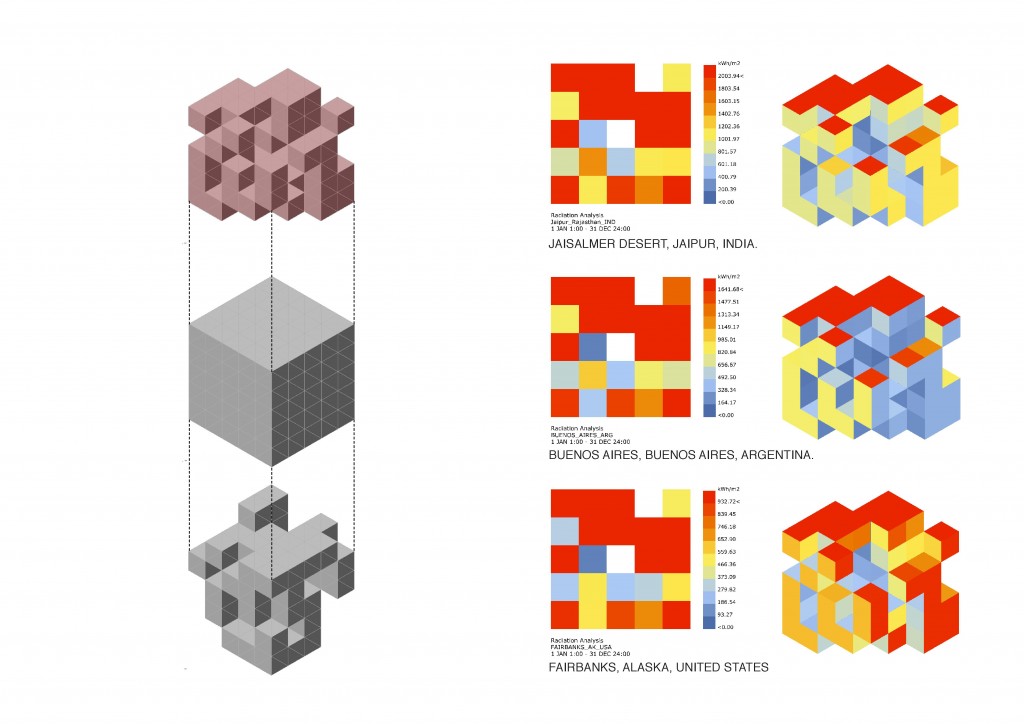
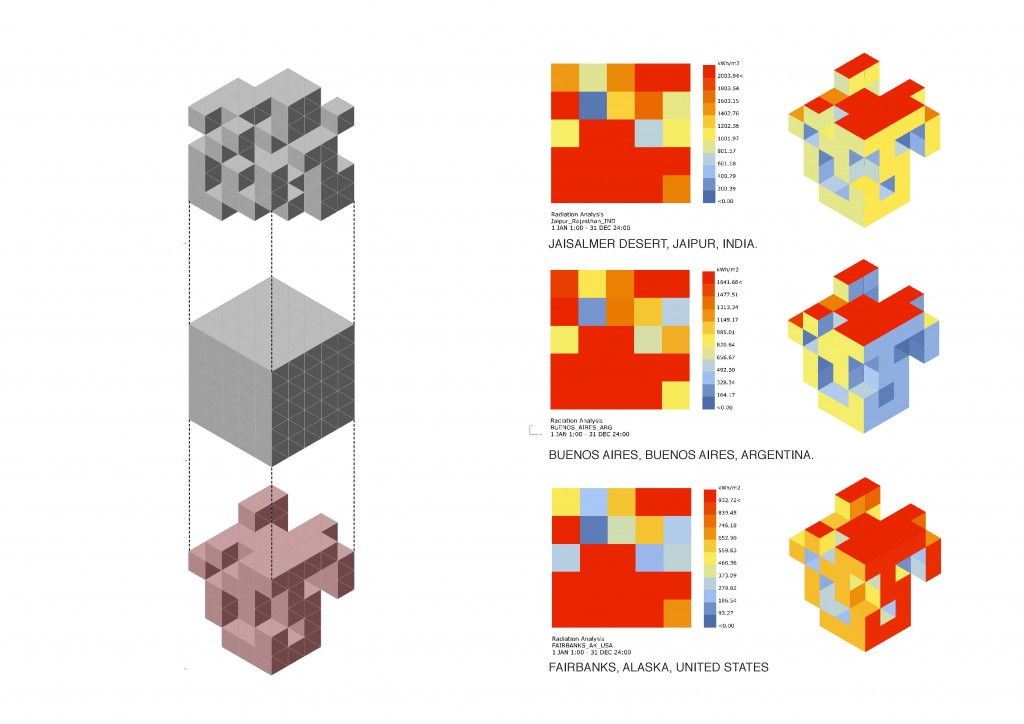
Nowadays, ecological awareness is increasing and human beings are looking for strategies to reduce the global warming impact; Figure 1 evidences the accelerated increase in the level of CO2 during the last 65 years.
Architects are engaged in projecting architecture that suggests the reduction of environmental impact of buildings by focusing on energy efficiency and ecological design. But is this approach enough to make a difference on a global scale? Is it possible to reduce global warming impact or will it continue increasing? Timothy Morton argues that 75% of the greenhouse gases nowadays present in the atmosphere will continue to be present in half a millennium; it will take around twenty-five thousand years for the oceans to absorb these gases.
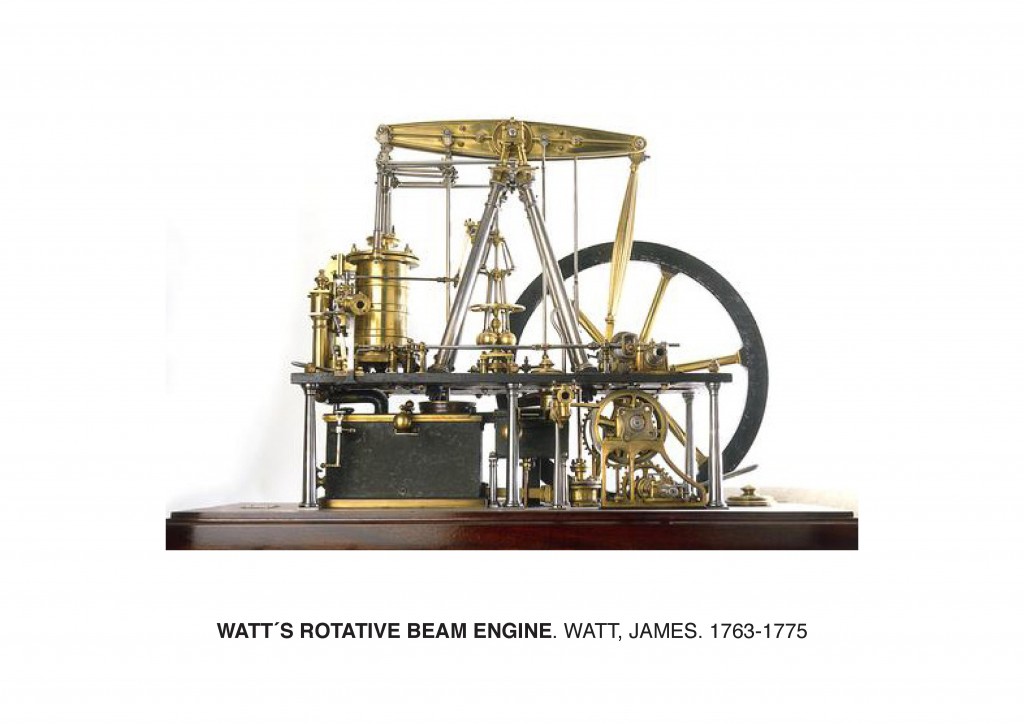
But when did human’s impact on the Earth begin? The renowned atmospheric chemist Paul J. Crutzen suggested that global warming had its origin at the beginning of Industrial Revolution; he defined this period as the Anthropocene Era. But what is the Anthropocene? In the year 2000, in IGBP newsletter 41, Crutzen expressed: “To assign a more specific date to the onset of the ‘anthropocene’ seems somewhat arbitrary, but we propose the latter part of the 18th century, although we are aware that alternative proposals can be made (some may even want to include the entire holocene). However, we choose this date because, during the past two centuries, the global effects of human activities have become clearly noticeable. This is the period when data retrieved from glacial ice cores show the beginning of a growth in the atmospheric concentrations of several “greenhouse gases”, in particular CO2 and CH4. Such a starting date also coincides with James Watt’s invention of the steam engine in 178410.”
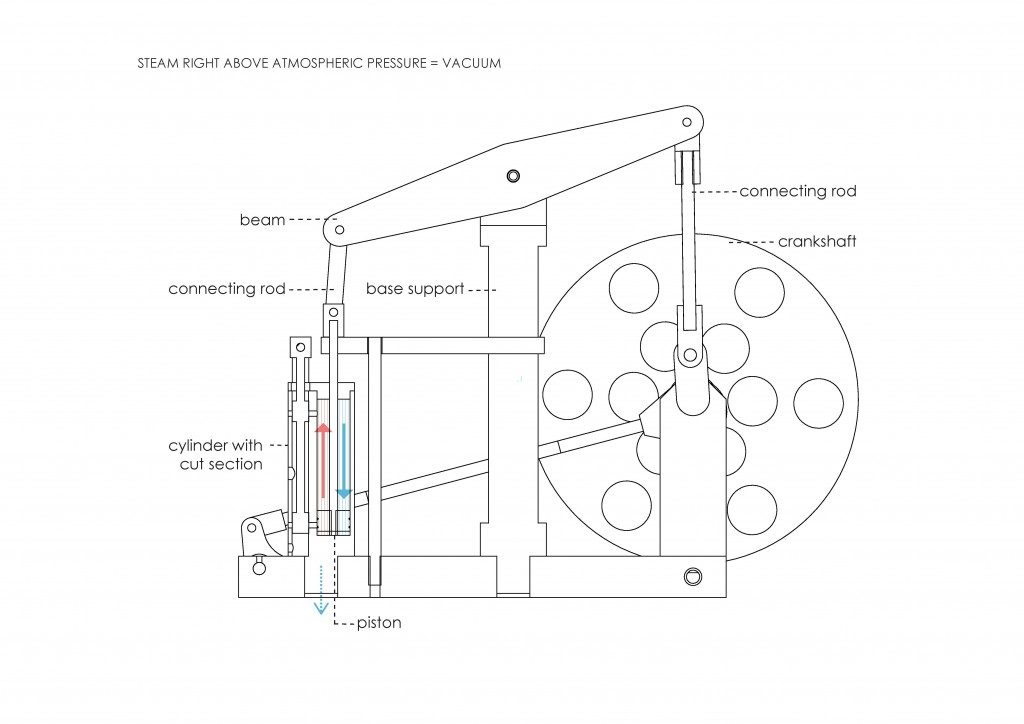
During the Industrial Revolution, there was an increase in the use of steam power thanks to the invention of the atmospheric engine. The first steam engine was developed by Thomas Newcomen in 1712. In 1859, physicist John Tyndall proved that water vapor and other gases were strongly absorbing infrared radiaton and thus generating a greenhouse effect. He stated: “This aqueous vapour is a blanket more necessary to the vegetable life of England than clothing is to man11”. His experiments showed that molecules of water vapor, carbon dioxide, and ozone were the top absorbers of heat radiation, and that even in reduced quantities, these gases absorbed more strongly than the atmosphere itself.
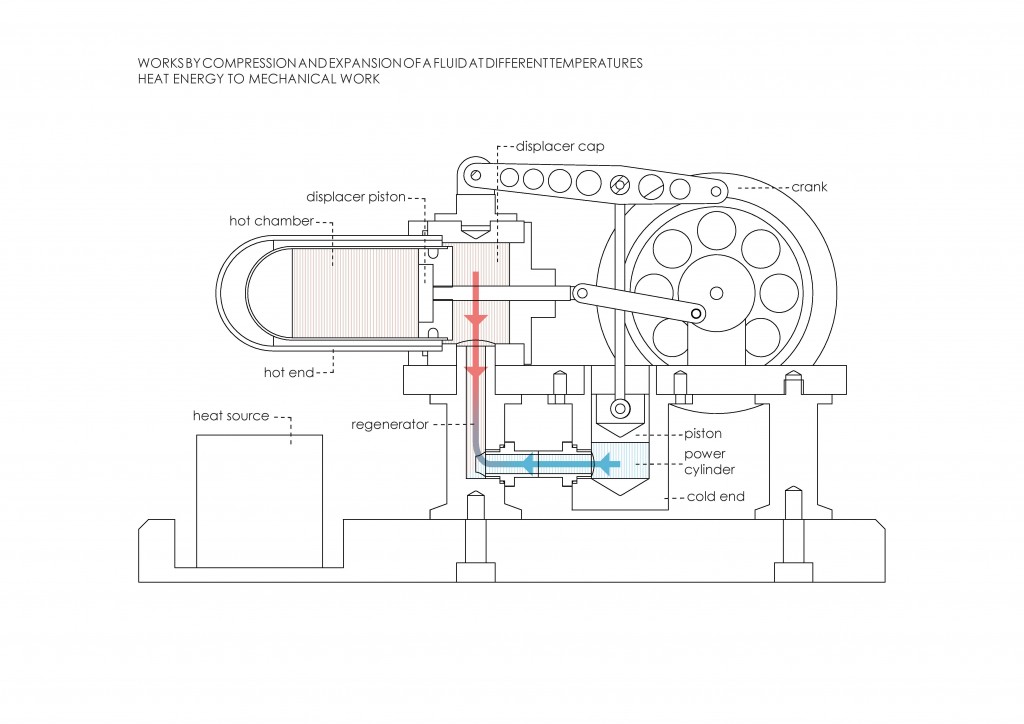
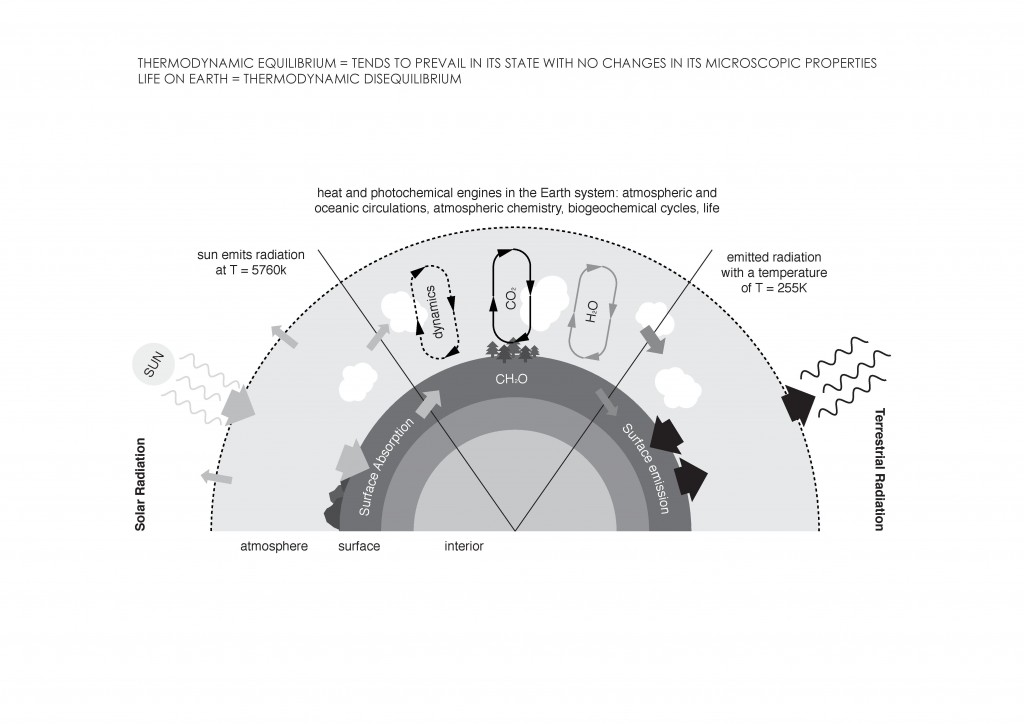
Before the Industrial Revolution, the idea of thermodynamics was not being developed. Its concept arose from the interest in increasing the steam engine´s efficiency. Nicolas Léonard Sadi Carnot, physicist and military engineer, considered that engine efficiency was crucial in order to win the Napoleonic Wars. He considered a reversible cyclic process made up of two isothermal and two adiabatic stages. This cycle is known as Carnot Cycle (Figure 2). Later, in 1851, Lord Kelvin became the first person to describe a heat pump, and in 1854 he formulated a definition of the term thermodynamics. “Thermo-dynamics is the subject of the relation of heat to forces acting between contiguous parts of bodies, and the relation of heat to electrical agency12.” It can also be defined as the branch of physics that deals with heat and temperature, and their relation to energy and work.
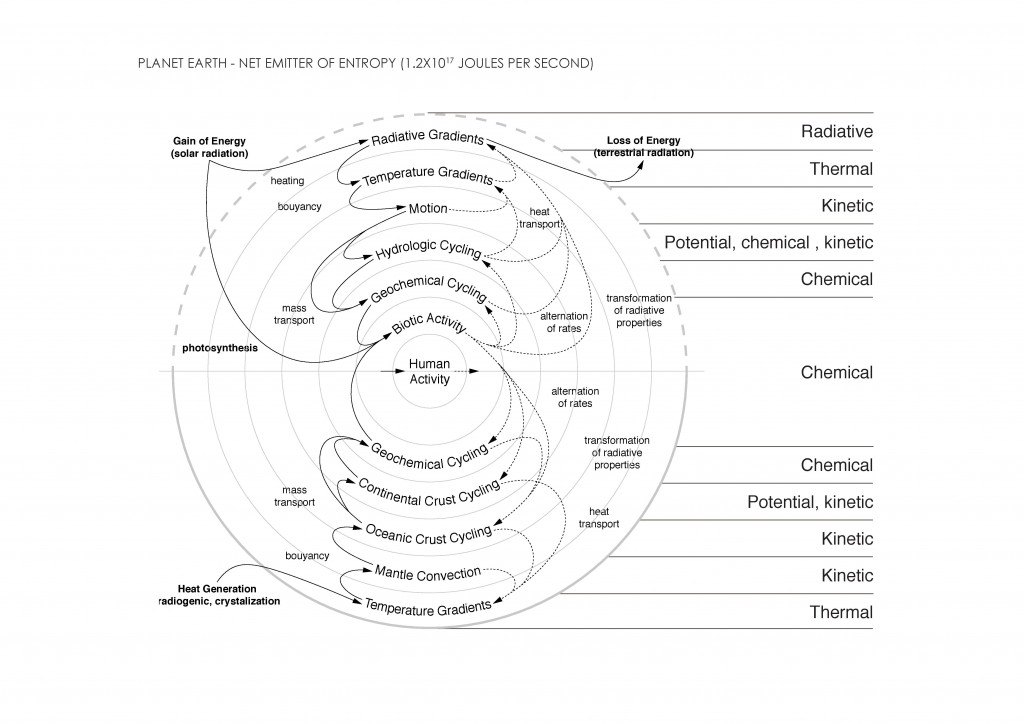
The main concepts of thermodynamics are energy, entropy, and equilibrium. Energy can be defined as the power resulting from the use of physical or chemical resources, especially to provide light and heat or to work machines13. Entropy can be understood as a measure of disorder within a macroscopic system.The entropy of the system is not measured in absolute terms; rather it is measured in relative terms. The entropy of the system is measured in terms of the changes the system has undergone from the previous state to the final state14. Equilibrium or thermodynamic equilibrium is the condition which a system, if left undisturbed, will approach over time. A system in thermodynamic equilibrium is in:
• mechanical equilibrium: no unbalanced forces
• thermal equilibrium: no temperature difference between parts in thermal contact
• chemical equilibrium: no net movement of material from one place or form to another
Reversibility is another key element in thermodynamics. A thermodinamically reversible process can be defined as “one such that the system can be restored to its initial state without any net change in the rest of the universe15.” Irreversible processes involve a net increase in the total entropy of the systems involved. The four laws of thermodynamics define fundamental physical quantities that identify thermodynamic systems at thermal equilibrium.
The Zeroth Law states that if two systems are separately in thermal equilibrium with a third, then they must be in thermal equilibrium with each other. If A=C, and B=C, then A=B.
The First Law states that energy is conserved; the amount of work required to change the state of a thermally isolated system depends solely on the initial and final states. Energy can be exchanged between physical systems as heat and work.
The Second Law presents several ways to define it. It can be stated according to Clausius, Kelvin, Caratheodory, and Entropy. The entropy statement defines that the total entropy can never decrease over time for an isolated system, that is, a system in which neither energy nor matter can enter nor leave.
The Third Law or Planck-Simon statement states that the contribution to the entropy of a system by each aspect of the system which is in internal thermodynamic equilibrium tends to zero as the temperature tends to absolute zero.
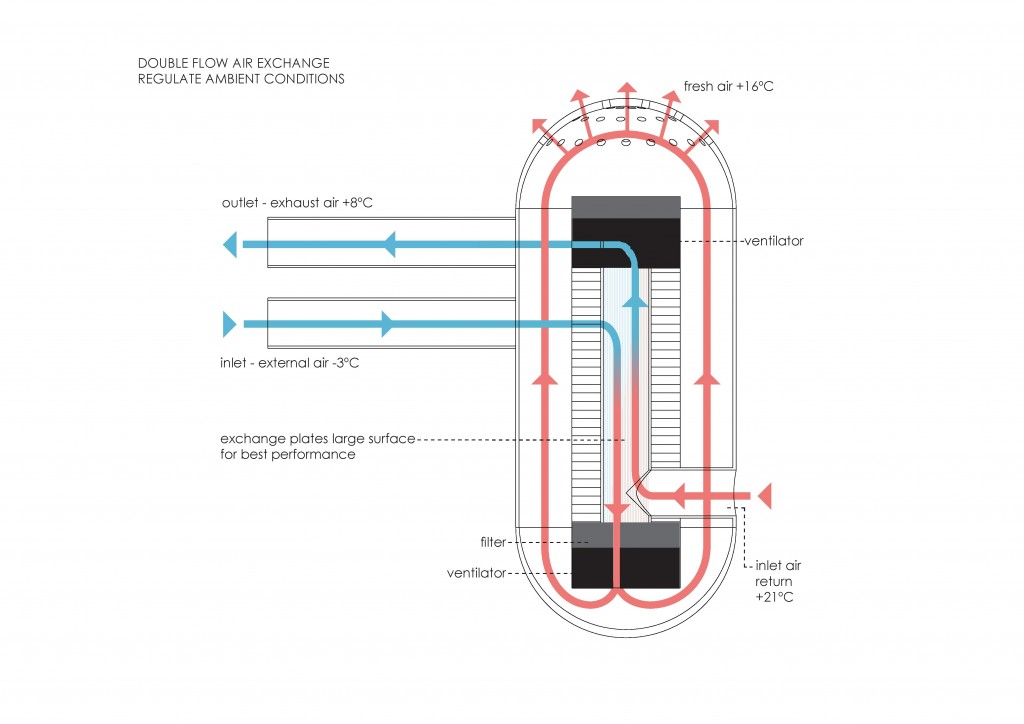
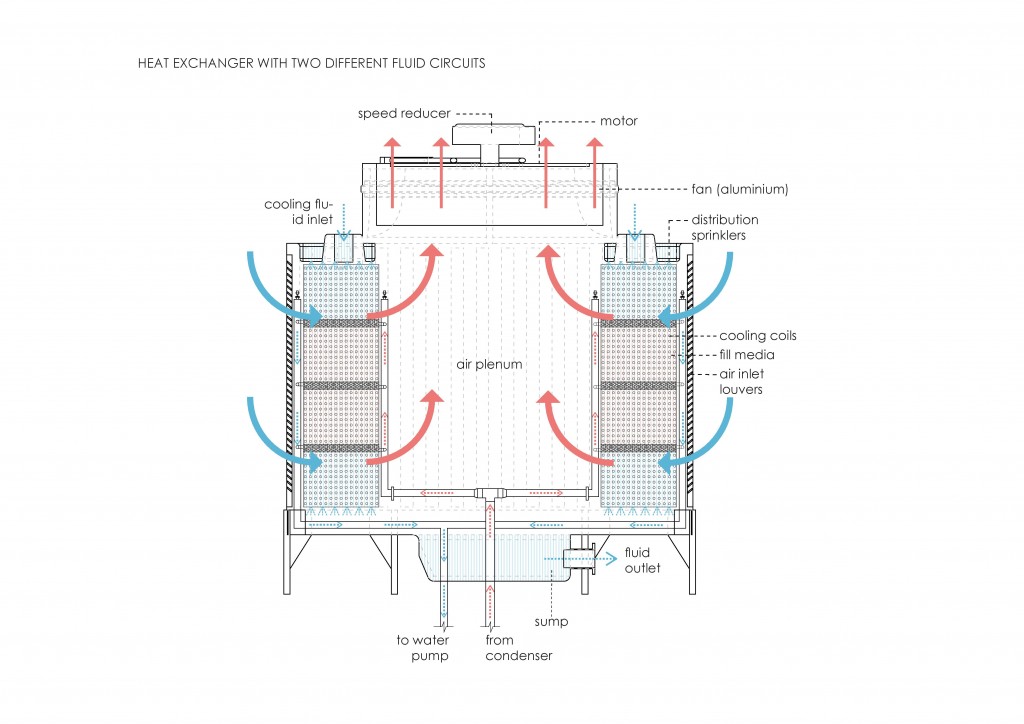
From the first law, heat transfer can be defined into various mechanisms such as thermal conduction, thermal convection, and thermal radiation.
Thermal conduction can be defined as the transfer of heat by means of molecular agitation within a material without any motion of the material as a whole.
Thermal convection can be defined as the transfer of heat by mass motion of a fluid such as air or water when the heated fluid is caused to move away from the source of heat, carrying energy with it. Convection above a hot surface occurs because hot air expands, becomes less dense, and rise16. The same happens with hot water, being less dense than cold water and, as a consequence, rising causing “convection currents which transport energy17”.
Thermal radiation is a “process by which energy, in the form of electromagnetic radiation, is emitted by a heated surface in all directions and travels directly to its point of absorption at the speed of light; thermal radiation does not require an intervening medium to carry it18.”
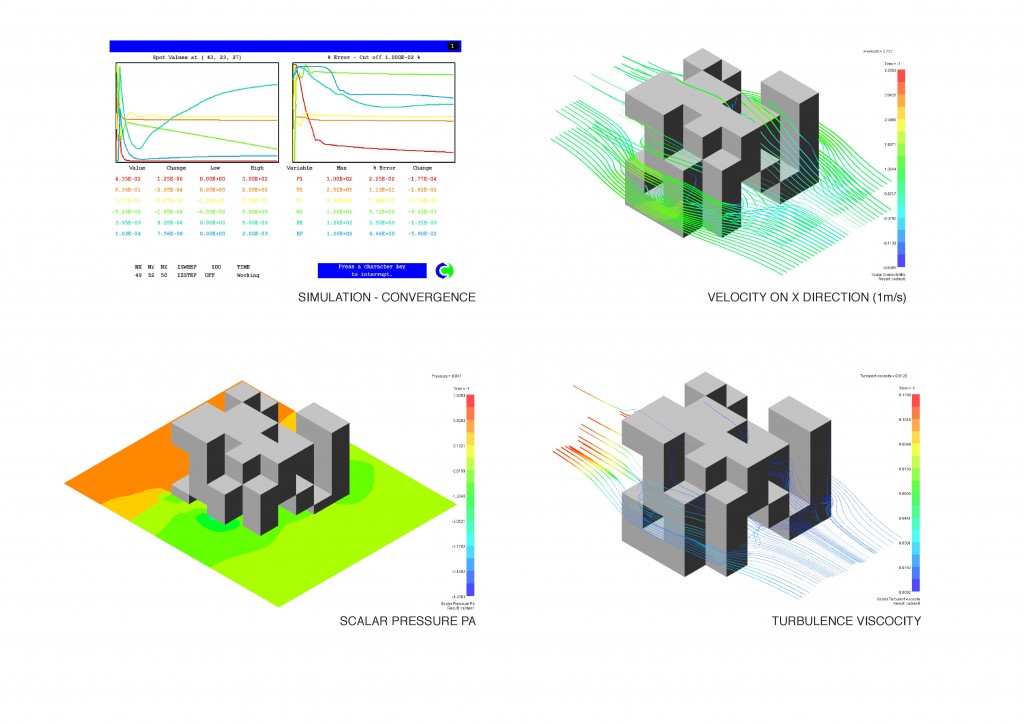
All these thermodynamic concepts are essential for the analysis of Global Warming in the condition of evolutionary process, defining technological strategies to design a system capable of altering the inmediate environment, and projecting the conditions of the future scenarios.